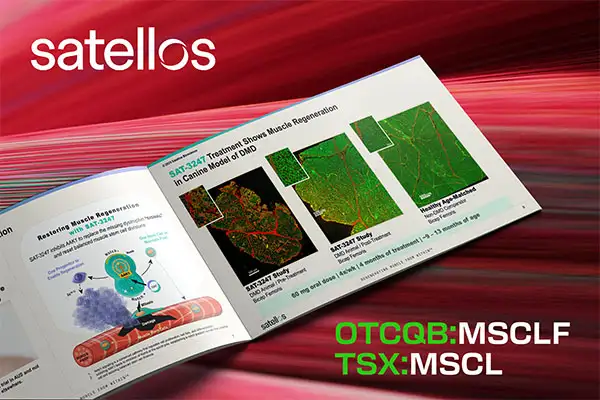
- BIOTECHNOLOGY
- October 5, 2025
- Editorial Feature
Small Molecule Innovation Opens New Pathways In Regenerative Medicine
From discovery to Phase 2 readiness in less than a decade, Satellos Bioscience Inc. (OTCQB:MSCLF, TSX:MSCL) is advancing what existing therapies have yet to achieve - the potential to restore the body’s ability to regenerate muscle and improve functional abilities lost from Duchenne muscular dystrophy (DMD), with a daily pill, SAT-3247.
“Satellos has the potential to produce substantial rewards if SAT-3247 can demonstrate sufficient muscle regeneration in DMD patients in a safe and tolerable manner.” i
Modern medicine has pushed the boundaries of what once seemed impossible. Scientists can now edit genes with CRISPRii, reprogram the immune system with CAR-T therapiesiii, and even grow replacement organs outside the body from engineered stem cells.vi These breakthroughs show just how far healthcare has advanced.
And yet, for all these advances, more than 95% of rare diseases still lack an FDA approved treatment.v Families facing these diagnoses are left with few answers or options.
DMD is one of the most devastating of examples. A genetic disease in which the dystrophin gene is mutated, DMD predominantly affects boys. It progressively damages, weakens and ultimately destroys every skeletal muscle in the body: first the legs, then the arms, and eventually the heart and lungs.vi
Children living with DMD are relentlessly robbed of functional ability, often confined to wheelchairs before their teens with their lives cut tragically short.
In the U.S. alone, more than 12,000 people live with Duchenne.That’s roughly 1 in every 3,500 male births.vii Despite billions in R&D, existing therapies can only stabilize muscle, often with serious side effects. No treatment has yet been designed to regenerate lost muscle.
This is where Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL) is breaking new ground. Its lead drug candidate, SAT-3247, represents the world’s first oral therapy in clinical development specifically designed to reset the body’s ability to regenerate muscle. In doing so, Satellos’ novel treatment aims to improve functional ability.
Early Phase 1 trials have already shown encouraging results with meaningful functional improvements, and Satellos is at a critical juncture as it prepares for pediatric Phase 2 studies. And it’s accomplished this at a pace that cuts the traditional biotech timeline nearly in half.viii
How SAT-3247 Targets Muscle Regeneration in DMD

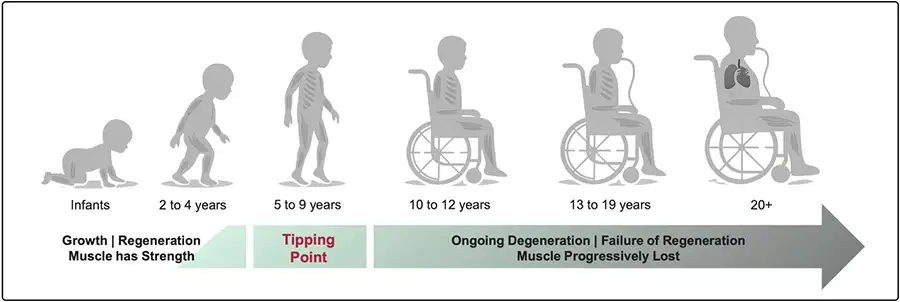
Early physical symptoms of DMD often appear between ages 2 and 4.
By ages 5 to 9, the disease reaches a tipping point: ongoing muscle damage begins to outpace the body’s ability to repair itself, locking patients into an irreversible, deadly decline. By ages 10 to 12, most boys lose the ability to walk, and often will not live beyond their twenties.ix
For decades, the standard of care has been corticosteroids, drugs that can slow progression but bring punishing side effects, from diabetes to osteoporosis to Cushing’s disease.x
Newer, largely genetically-based therapies have shown incremental benefits. However, they also often come with severe side effects. And, they can only delay decline. Critically, none has shown the ability to regenerate lost muscle!
Satellos’ scientific founder was first to challenge prevailing approaches and look deeper. Dr. Michael Rudnicki and his team questioned the long-held view that Duchenne is solely a disease of muscle damage. Their discoveries have revealed that the problem of relentless muscle loss and functional decline lies in the body’s innate repair system.xi
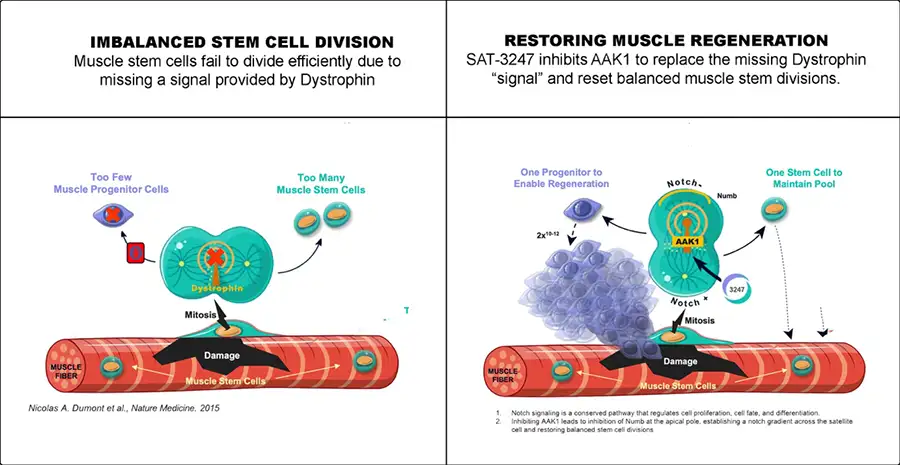
Healthy muscle depends on muscle stem cells for repair. These specialized cells exist to create new muscle cells in response to damage. In a process known as asymmetric division, two differentiated cells are created from each dividing stem cell: one cell becomes a muscle progenitor cell to go on to repair damage, while the other remains a stem cell to preserve long-term regenerative ability.
However, in DMD – where muscle is constantly being damaged – the process of asymmetric division to create new muscle is severely impaired. Thus, ongoing repair falls behind ongoing damage leading to progressive muscle loss and functional decline.
Dr. Rudnicki discovered, and published in the peer reviewed scientific journal Nature Medicine, that dystrophin provides a biochemical ‘signal’ in healthy muscle stem cells to orchestrate the process of asymmetric division.xii In DMD, this protein – and hence the biochemical ‘signal’ is missing. Muscle stem cells in people living with DMD cannot make enough new progenitor muscle cells to keep up with ongoing damage.xiii
As a result, regeneration collapses. The body simply cannot keep pace with the relentless damage caused by DMD. Over time, muscle is progressively destroyed and functional ability lost.
Satellos’ lead drug candidate, SAT-3247, has been designed to replace that missing biochemical ‘signal’. Satellos discovered that by inhibiting a different protein also found in muscle stem cells, called AAK1, asymmetric division and muscle repair can be rebooted, effectively turning the body’s natural repair machinery back on.xiv
This marks a major turning point. While other approaches pursue mutation-specific fixes or address a selected age group, thereby excluding many patients, SAT-3247 targets a universal root cause of Duchenne. That means it has the potential to benefit all people living with DMD, regardless of their genetic profile or age.
And, unlike existing therapies that stabilize or slow decline, Satellos’ approach is designed to regenerate muscle to improve strength and functional ability. Results in our Phase 1b clinical trials in adults with DMD provide encouragement to us that this may be possible.
Phase 1b: Average Grip Strength Nearly Doubled in 28 Days

Drug development is usually a long game – averaging 10–15 years from discovery to FDA approval.xv Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL) is working to compress that timeline by advancing with urgency, but also with the care needed to avoid setbacks.
That discipline is already paying off. SAT-3247 has cleared its first clinical hurdle in Phase 1 with results that mark tangible progress.
In Phase 1a, 72 healthy volunteers confirmed the drug’s safety profile. Taken once daily, SAT-3247 cleared from the body within 24 hours, as intended, and showed no serious side effects.xvi
Even more telling data came from Phase 1b, where five adults with Duchenne, aged 20–27, received treatment with SAT-3247 for 28 days. At this advanced disease stage, grip strength is often the last measurable function left.
Here, SAT-3247 delivered a result few would have thought possible. Average grip strength nearly doubled, rising from 1.9kg to 3.9kg: a 2kg average gain in strength in under a month. Patients also recorded an average 5% improvement in lung function.xvii
For context, even stabilizing function in such advanced patients is considered a success. To see measurable improvements in strength, and so quickly, is unprecedented.
The comparison with peers is striking. Wave Life Sciences, a major player in the DMD space with a market cap of ~$1.2 billionxviii, publicly reported a 1kg improvement in grip strength after 48 weeks of treatment in younger patients averaging 8 years old.xvii
By contrast, Satellos achieved twice the improvement in one-seventeeth the time, and in older, more advanced patients.
And while competing therapies often require complex infusions, SAT-3247 is a once-daily pill. This simplified approach reduces treatment burden, lowers COGS, supports worldwide scalability, and provides attractive competitive advantages.
The Next Milestone: Pediatric Phase 2 Trials
Satellos’ preclinical models showed strength returning and compounding over time.xx
To test whether similar patterns can be observed in humans, adult participants from the Phase 1b study have been invited to re-enroll in an 11-month extension study, LT-001. Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL) hopes to enroll additional DMD adults and has filed for approval to expand the trial.xxi
In addition to the LT-001 trial, Satellos is filing regulatory submissions in multiple countries around the world, in addition to the US, in order to conduct a Phase 2 clinical trial in children with DMD.xxii
Subject to approvals, the Company plans to initiate this trial in 2025. Designed as a randomized, placebo-controlled study with two treatment arms of differing dose levels, CL-201 proposes to enroll up to 51 ambulatory boys in a 3-month study. Biomarkers, muscle composition (histology and MRI) and multiple functional endpoints will be measured. Unlike peer trials often running 12 to 18 months to show potential for benefit, Satellos has chosen 3-months based on timing of results seen in its preclinical studies and further supported by the results seen in the Phase 1b trial.xxiii
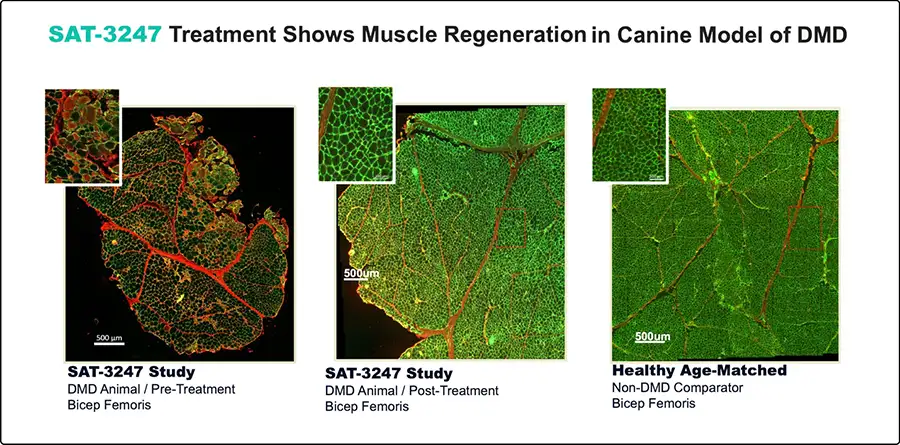
Satellos has the resources to execute. With US$38 million in cash, the Company has operational financing through 2026 — sufficient to support completion of Phase 2 without near-term financing risk. xxiv Management is also evaluating a potential Nasdaq uplist as progress is made and conditions allow, to ensure maximum flexibility to optimize SAT-3247.xxv
Ownership is also tightly held. Insiders control 15% of shares, while more than 50% are backed by healthcare-focused institutional investors.
A Growing Field with Multi-Billion-Dollar Peers
The market for Duchenne therapies is accelerating to meet urgent patient need. Valued at $4.1 billion in 2024, it is projected to nearly quintuple to $19.5 billion by 2034.xxvi That growth has already drawn heavy investor interest, with peer companies commanding multi-billion-dollar valuations:
Companies such as Sarepta Therapeutics (Nasdaq: SRPT; ~$1.8B market cap)xxvii, Dyne Therapeutics (Nasdaq: DYN; ~$1.9B market cap)xxvii, Avidity Biosciences (Nasdaq: RNA; ~$6.0B market cap), and WVE (~$1.2B market cap) are all pursuing DMD treatment programs. Most of these approaches have been designed to stabilize muscle and are specific to certain genetic subsets of patients or limited by age.
By contrast, Satellos has designed its therapy to have the potential to be taken by all patients. This potential for universal scope, combined with preclinical and early Phase 1 data showing functional improvements, positions Satellos apart from its peers.
Satellos Leadership and Scientific Team

Michael Rudnicki, OC, Ph.D., FRS, FRSC – Co-founder & Chief Discovery Officer
Dr. Michael Rudnicki is recognized globally as a pioneer in muscle stem cell biology. His landmark discoveries transformed the understanding of skeletal muscle and Duchenne muscular dystrophy. In a 2007 Cell paper, he was the first to define and characterize a true population of multipotent muscle stem cells. In 2015, his team uncovered dystrophin’s critical role in orienting these cells for asymmetric division, reframing Duchenne not just as a disease of fragile muscle fibers, but as a disease of failed muscle regeneration.
That insight underpins Satellos’ approach and the development of SAT-3247. A Fellow of the Royal Society and Officer of the Order of Canada, Rudnicki brings both scientific authority and visionary leadership.

Frank Gleeson, MBA – Co-founder, Chief Executive Officer & Board Director
Frank Gleeson has spent his career turning early-stage science into funded, scalable businesses. Throughout his career, he has been an operational executive, venture-capitalist, biotech CEO and now, an entrepreneur. Mr. Gleeson has built more than 20 biomedical companies and negotiated over $500 million in financings and M&A transactions.
Before co-founding Satellos in 2018 with Dr. Rudnicki, the two of them teamed up to found Verio Therapeutics in 2008 which they successfully exited into Fate Therapeutics and became part of the team until 2015. This experience gave them the fire to work together again!
Before his entrepreneurial career, Frank held senior roles with CPDC, FACIT, and MDS Capital, where he oversaw a $250 million fund focused on drug discovery ventures. His operational roots include a 17-year career with ICI plc, which eventually went on to become AstraZeneca.

Elizabeth Williams, CPA, CA – Chief Financial Officer
With nearly 20 years in biotech finance, Elizabeth Williams ensures Satellos has the structure and runway to deliver on its milestones. She previously served as CFO of Medicenna, where she oversaw its uplist from TSXV to Nasdaq, and as VP of Finance at Aptose Biosciences. Her experience spans financings, investor relations, and regulatory compliance. Williams’ expertise ensures capital discipline matches scientific discipline, a critical factor in compressing timelines without compromising efficacy.

Wildon Farwell, M.D. – Chief Medical Officer
Dr. Wildon Farwell brings a rare combination of clinical credibility and regulatory experience. At Biogen, he led late-stage development of Spinraza, the first FDA-approved treatment for spinal muscular atrophy, as well as played key roles in ALS program development. As CMO at Dyne Therapeutics, he guided Duchenne and myotonic dystrophy programs through clinical trials. His deep relationships with regulators and clinicians ensure Satellos’ clinical path is designed for both speed and approvability.

Phil Lambert, Ph.D. – Chief Scientific Officer
Dr. Phil Lambert has advanced more than 20 therapies into the clinic, with 25+ years of drug discovery and development experience spanning neuroscience, metabolic, inflammatory, orphan, and ocular diseases. A neuroscientist by training, he has led programs across academia, biotech, pharma, and CROs, including at Regeneron, GlaxoSmithKline, and the ALS Therapy Development Institute. He also co-founded and grew VivoPath before its acquisition by Charles River.
Built on a Foundation of Patient Support
When it comes to rare diseases like Duchenne muscular dystrophy, progress doesn’t happen without the support of patient foundations and advocacy networks. These organizations fund early-stage science, shape trial design, and ensure that drug development aligns with the realities families face every day.
For Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL), this support goes well beyond collaboration Parent Project Muscular Dystrophy (PPMP) – a leading patient advocacy group founded by parents of children with DMD – was an early financial supporter of Satellos. That support has been instrumental in enabling the company to establish the discovery program leading to the invention of SAT-3247. PPMD also helped ensure that Satellos’ development path is grounded in what matters most to families: not just extending timelines but restoring function and quality of life.
When combined with the backing of the Canadian Stem Cell Networkxxix and other research foundations, Satellos benefits from a unique ecosystem: one where patients, scientists, and investors are aligned around the same goal.
This alignment of patients, scientists, and investors has enabled SAT-3247 to rapidly progress from discovery into clinical studies and provides important networks to build on for the future.
- First Potential Muscle Regeneration Therapy: SAT-3247 is designed to regenerate lost muscle tissue rather than just slow disease progression, representing a paradigm shift from all existing DMD treatments that only manage symptoms.
- Superior Clinical Results in Unprecedented Timeframe:: Phase 1b trials showed grip strength nearly doubling in 28 days, while competitor therapies required nearly a year to achieve much smaller improvements in younger, less-advanced patient populations.
- Durability Potential Beyond Early Gains: Preclinical models showed strength improvements compounding over months, and Satellos is now following adult patients in an 11-month trial to demonstrate long-term restoration.
- Unmet Need Offers Unique Opportunity With Orphan Drug and Rare Pediatric Disease designations in hand, Satellos is well positioned to capitalize if Phase 2 results show functional improvement, potentially compressing traditional approval timelines.
- Universal Treatment Approach: Unlike competitors targeting specific genetic mutations or age ranges, SAT-3247 is intended to work across all DMD mutation types or stages of development, addressing all genetic subsets and age groups.
- Global Scalability Through Oral Delivery: SAT-3247 is a simple daily pill while competing therapies require complex hospital infusions, eliminating barriers and enabling worldwide patient access.
- Multiple Catalysts Over Next 12 Months: Phase 2 pediatric trials planned to start in late 2025 with 51 patients in a 3-month study, plus potential Nasdaq uplist, create the potential for multiple milestones.
7 Reasons Why You Should Consider Adding Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL) to Your Watchlist
Satellos Bioscience (OTCQB:MSCLF, TSX:MSCL) has advanced from discovery to Phase 2 planning in less than a decade, advancing a novel therapy designed to target muscle regeneration in Duchenne — a completely different approach compared with treatments designed only to stabilize muscle. SAT-3247 has the potential to restore lost muscle for Duchenne patients worldwide. Backed by breakthrough science published in top peer-reviewed journals, an experienced leadership team, foundation support, and a strong funding base, the company is at a pivotal juncture in moving SAT-3247 forward.
To learn more about Satellos’ science, pipeline, and current studies, visit satellos.com. And if you’d like to be first to receive news and updates as they happen, enter your email below.
Start Tracking Satellos Bioscience - Add It To Your Watch List Today!
ii https://www.asbmb.org/asbmb-today/science/111724/crispr-gene-editing-moving-closer-to-home
iii https://www.uchicagomedicine.org/forefront/immunotherapy-articles/car-t-cell-therapy-treating-autoimmune-diseases
iv https://www.ucsf.edu/news/2024/12/429211/scientists-take-first-steps-toward-growing-organs-scratch
v https://ncats.nih.gov/sites/default/files/NCATS_RareDiseasesFactSheet.pdf
vi https://www.ninds.nih.gov/health-information/disorders/muscular-dystrophy
vii https://seekingalpha.com/article/4818495-satellos-bioscience-promising-novel-treatment-for-duchenne-muscular-dystrophy
viii https://pmc.ncbi.nlm.nih.gov/articles/PMC3058157/
ix https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 5
x https://www.medicalnewstoday.com/articles/corticosteroids
xi https://www.nature.com/articles/npjregenmed20166
xii https://www.nature.com/articles/npjregenmed20166
xiii https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 7
xiv https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 7
xv https://pmc.ncbi.nlm.nih.gov/articles/PMC3058157/
xvi https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 12
xvii https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 14
xviii https://companiesmarketcap.com/gbp/sarepta-therapeutics/marketcap/#google_vignette
xix https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 15
xx https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 9
xxi https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 17
xxii https://ir.satellos.com/news/news-details/2024/Satellos-Announces-Submission-of-Regulatory-Filing-to-Commence-a-Phase-1-Clinical-Trial-with-SAT-3247/default.aspx
xxiii https://s203.q4cdn.com/439234936/files/doc_presentations/2025/Aug/07/Satellos-Non_Con_Deck_8-06-25.pdf, page 17
xxiv https://ir.satellos.com/news/news-details/2024/Satellos-Announces-Closing-of-US40M-Public-Offering/default.aspx
xxv xxvi https://www.precedenceresearch.com/duchenne-muscular-dystrophy-drugs-market
xxvii https://companiesmarketcap.com/gbp/sarepta-therapeutics/marketcap/#google_vignette
xxviii https://finance.yahoo.com/quote/DYN/
xxix https://stemcellnetwork.ca/knowledge-mobilization/companyspotlights/
IMPORTANT NOTICE AND DISCLAIMER
This article is a paid advertisement. Think Ink Marketing and its owners, managers, employees, and assigns (collectively “the Publisher”) is often paid by profiled companies or third parties to organize marketing campaigns, which include the creation and dissemination of these types of communications. In this case, in an effort to enhance public awareness of for Satellos Bioscience Inc. (“MSCL”) and its securities, MSCL has provided the Publisher with a budget of approximately $10,000 USD to cover the costs associated with the creation and distribution of this communication. The Publisher may retain any excess sums after expenses as its compensation. This compensation should be viewed as a major conflict with our ability to be unbiased. Readers should beware that third parties, profiled companies, and/or their affiliates may liquidate shares of the profiled companies at any time, including at or near the time you receive this communication, which has the potential to hurt share prices. Frequently companies profiled in our articles experience a large increase in volume and share price during the course of investor awareness marketing, which often ends as soon as the investor awareness marketing ceases. The investor awareness marketing may be as brief as one day, after which a large decrease in volume and share price may likely occur. This communication is not, and should not be construed to be, an offer to sell or a solicitation of an offer to buy any security. Neither this communication nor the Publisher purport to provide a complete analysis of any company or its financial position. The Publisher is not, and does not purport to be, a broker-dealer or registered investment adviser. This communication is not, and should not be construed to be, personalized investment advice directed to or appropriate for any particular investor. Any investment should be made only after consulting a professional investment advisor and only after reviewing the financial statements and other pertinent corporate information about the company. Further, readers are advised to read and carefully consider the Risk Factors identified and discussed in the advertised company’s SEC, SEDAR and/or other government filings. Investing in securities, particularly microcap securities, is speculative and carries a high degree of risk. Past performance does not guarantee future results. This communication is based on information generally available to the public and on interviews with company management, and does not (to the Publisher’s knowledge, as confirmed by MSCL) contain any material, non-public information. The information on which it is based is believed to be reliable. Nevertheless, the Publisher cannot guarantee the accuracy or completeness of the information.
SHARE OWNERSHIP.
The Publisher does not own any shares of MSCL and has no information concerning share ownership by others of in MSCL. The Publisher cautions readers to beware that third parties, profiled companies, and/or their affiliates may liquidate shares of the profiled companies at any time, including at or near the time you read the articles on this website and this has the potential to hurt share prices. Frequently companies profiled in such articles experience a large increase in volume and share price during the course of investor awareness marketing, which often ends as soon as the investor awareness marketing ceases.
FORWARD LOOKING STATEMENTS.
This publication contains forward-looking statements, including statements regarding expected continual MSCLwth of the featured companies and/or industry. The Publisher notes that statements contained herein that look forward in time, which include everything other than historical information, involve risks and uncertainties that may affect the companies’ actual results of operations. Wherever possible, words such as “predicts”, “projects”, “targets”, “plans”, “expects”, “does not expect”, “budget”, “scheduled”, “estimates”, “forecasts”, “anticipate” or “does not anticipate”, “believe”, “intend” and similar expressions or statements that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, or the negative or grammatical variation thereof or other variations thereof, or comparable terminology have been used to identify forward-looking statements. These forward-looking statements include, among other things, statements relating to: (a) revenue generating potential with respect to MSCL’s industry; (b) market opportunity; (c) MSCL’s business plans and strategies; (d) services that MSCL intends to offer; (e) MSCL’s milestone projections and targets; (f) MSCL’s expectations regarding receipt of approval for regulatory applications; (g) MSCL’s intentions to expand into other jurisdictions including the timeline expectations relating to those expansion plans; and (h) MSCL’s expectations regarding its ability to deliver shareholder value. Forward-looking statements are not a guarantee of future performance and are based upon a number of estimates and assumptions of management in light of management’s experience and perception of trends, current conditions and expected developments, as well as other factors that management believes to be relevant and reasonable in the circumstances, as of the date of this document including, without limitation, assumptions about: (a) the ability to raise any necessary additional capital on reasonable terms to execute MSCL’s business plan; (b) that general business and economic conditions will not change in a material adverse manner; (c) MSCL’s ability to procure equipment and operating supplies in sufficient quantities and on a timely basis; (d) MSCL’s ability to enter into contractual arrangements; (e) the accuracy of budgeted costs and expenditures; (f) MSCL’s ability to attract and retain skilled personnel; (g) political and regulatory stability; (h) the receipt of governmental, regulatory and third-party approvals, licenses and permits on favorable terms; (i) changes in applicable legislation; (j) stability in financial and capital markets; and (k) expectations regarding the level of disruption as a result of COVID-19. Such forward-looking information involves a variety of known and unknown risks, uncertainties and other factors which may cause the actual plans, intentions, activities, results, performance or achievements of MSCL to be materially different from any future plans, intentions, activities, results, performance or achievements expressed or implied by such forward-looking statements. Such risks include, without limitation: (a) MSCL operations could be adversely affected by possible future government legislation, policies and controls or by changes in applicable laws and regulations; (b) public health crises such as the COVID-19 pandemic may adversely impact MSCL’s business; (c) the volatility of global capital markets; (d) political instability and changes to the regulations governing MSCL’s business operations (e) MSCL may be unable to implement its MSCLwth strategy; and (f) increased competition. Except as required by law, the Website Host undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future event or otherwise.
INDEMNIFICATION/RELEASE OF LIABILITY.
By reading this communication, you acknowledge that you have read and understand this disclaimer, and further that to the greatest extent permitted under law, you release the Publisher, its affiliates, assigns and successors from any and all liability, damages, and injury from this communication. You further warrant that you are solely responsible for any financial outcome that may come from your investment decisions.
INTELLECTUAL PROPERTY.
Think Ink Marketing is the Publisher’s trademark. All other trademarks used in this communication are the property of their respective trademark holders. The Publisher is not affiliated, connected, or associated with, and is not sponsored, approved, or originated by, the trademark holders unless otherwise stated. No claim is made by the Publisher to any rights in any third-party trademarks.
IMPORTANT NOTICE AND DISCLAIMER
This website is owned and hosted by Tycona Media Ltd. Articles appearing on this website should be considered paid advertisements. Tycona Media Ltd. and its owners, managers, employees, and assigns (collectively “the Website Host”) is often paid by marketing companies to host websites on which articles profiling public companies are published. The Website Host has not been compensated by any of the profiled companies. The Website Host’s compensation for articles appearing on this website is as follows:
The Website Host has been paid approximately $500 per month while the advertisement campaign is active by Think Ink Marketing as compensation to host the article profiling Satellos Bioscience Inc. (“MSCL”).
SHARE OWNERSHIP
The Website Host does not own any shares of any profiled compannies and has no information concerning share ownership by others of any profiled companies. The Website Host cautions readers to beware that third parties, profiled companies, and/or their affiliates may liquidate shares of the profiled companies at any time, including at or near the time you read the articles on this website and this has the potential to hurt share prices. Frequently companies profiled in such articles experience a large increase in volume and share price during the course of investor awareness marketing, which often ends as soon as the investor awareness marketing ceases.
NO SECURITIES OFFERED
The articles on this website are not, and should not be construed to be, offers to sell or solicitations of an offer to buy any security. Neither the articles on this website nor the Website Host purport to provide a complete analysis of MSCL or its financial position. The Website Host is not, and does not purport to be, a broker-dealer or registered investment adviser. The articles on this website are not, and should not be construed to be, personalized investment advice directed to or appropriate for any particular investor. Any investment should be made only after consulting a professional investment advisor and only after reviewing the financial statements and other pertinent corporate information about MSCL Further, readers are advised to read and carefully consider the Risk Factors identified and discussed in MSCL’s SEC, SEDAR and/or other government filings. Investing in securities, particularly microcap securities, is speculative and carries a high degree of risk.
INDEMNIFICATION/RELEASE OF LIABILITY
By reading articles on this website, you acknowledge that you have read and understood this disclaimer, and further that to the greatest extent permitted under law, you release the Website Host, its affiliates, assigns and successors from any and all liability, damages, and injury from articles appearing on this website. You further warrant that you are solely responsible for any financial outcome that may come from your investment decisions.
LINKS TO THIRD PARTY WEBSITES
This website enables users to link to external websites not under the control of The Website Host. The Website Host has no control over the nature, content, and availability of those sites. The inclusion of any links is not intended as, and should not be construed as, a recommendation or endorsement of the content or views expressed on such external websites. The Website Host expressly disclaims any representation concerning the quality, safety, suitability, or reliability of any external websites and the content and materials contained in them. It is important for users to take necessary precautions, especially to ensure appropriate safety.
INTELLECTUAL PROPERTY
The Tomorrow Investor is the Website Host’s trademark. All other trademarks used in this communication are the property of their respective trademark holders. The Website Host is not affiliated, connected, or associated with, and is not sponsored, approved, or originated by, the trademark holders unless otherwise stated. No claim is made by the Website Host to any rights in any third-party trademarks.
FORWARD LOOKING INFORMATION
This document contains forward-looking information and forward-looking statements, within the meaning of applicable Canadian securities legislation, (collectively, “forward-looking statements”), which reflect expectations regarding MSCL’s future growth, future business plans and opportunities, expected activities, and other statements about future events, results or performance. Wherever possible, words such as “predicts”, “projects”, “targets”, “plans”, “expects”, “does not expect”, “budget”, “scheduled”, “estimates”, “forecasts”, “anticipate” or “does not anticipate”, “believe”, “intend” and similar expressions or statements that certain actions, events or results “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, or the negative or grammatical variation thereof or other variations thereof, or comparable terminology have been used to identify forward-looking statements. These forward-looking statements include, among other things, statements relating to: (a) revenue generating potential with respect to MSCL’s industry; (b) market opportunity; (c) MSCL’s business plans and strategies; (d) services that MSCL intends to offer; (e) MSCL’s milestone projections and targets; (f) MSCL’s expectations regarding receipt of approval for regulatory applications; (g) MSCL’s intentions to expand into other jurisdictions including the timeline expectations relating to those expansion plans; and (h) MSCL’s expectations regarding its ability to deliver shareholder value. Forward-looking statements are not a guarantee of future performance and are based upon a number of estimates and assumptions of management in light of management’s experience and perception of trends, current conditions and expected developments, as well as other factors that management believes to be relevant and reasonable in the circumstances, as of the date of this document including, without limitation, assumptions about: (a) the ability to raise any necessary additional capital on reasonable terms to execute MSCL’s business plan; (b) that general business and economic conditions will not change in a material adverse manner; (c) MSCL’s ability to procure equipment and operating supplies in sufficient quantities and on a timely basis; (d) MSCL’s ability to enter into contractual arrangements; (e) the accuracy of budgeted costs and expenditures; (f) MSCL’s ability to attract and retain skilled personnel; (g) political and regulatory stability; (h) the receipt of governmental, regulatory and third-party approvals, licenses and permits on favorable terms; (i) changes in applicable legislation; (j) stability in financial and capital markets; and (k) expectations regarding the level of disruption as a result of COVID-19. Such forward-looking information involves a variety of known and unknown risks, uncertainties and other factors which may cause the actual plans, intentions, activities, results, performance or achievements of MSCL to be materially different from any future plans, intentions, activities, results, performance or achievements expressed or implied by such forward-looking statements. Such risks include, without limitation: (a) MSCL’s operations could be adversely affected by possible future government legislation, policies and controls or by changes in applicable laws and regulations; (b) public health crises such as the COVID-19 pandemic may adversely impact MSCL’s business; (c) the volatility of global capital markets; (d) political instability and changes to the regulations governing MSCL’s business operations (e) MSCL may be unable to implement its growth strategy; and (f) increased competition. Except as required by law, the Website Host undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future event or otherwise.
HISTORICAL INFORMATION
Any graphs, tables or other information demonstrating the historical performance or current or historical attributes of MSCL or any other entity contained in this document are intended only to illustrate historical performance or current or historical attributes of MSCL or such entities and are not necessarily indicative of future performance of MSCL or such entities.
COMPANY SPOTLIGHT